INTRODUCTION
Asexual reproduction is rare in most animal phyla. For thalaceans however, asexual reproduction is an integral element of their lifecycle (Gibson and Paffenhöfer, 2002). A common thalacean, Thalia democratica, is an important class of gelatinous zooplankton. Inhabiting continental shelf water globally, T. democratica swarms are generally short lived, but can reach magnitudes of up to 350 individuals / m3 (Henschke et al., 2011). The life cycle of T. democratica involves the asexual reproduction of multiple aggregates released as a chain. Each aggregate is internally fertilized andproduces one offspring before developing testes, releasing sperm, and dying (Kremer, 2002). Each solitary individual has the capacity to producelarge numbers of asexual buds in continuum, while aggregate individuals only have to capacity to sexually produce one individual. This identifies the importance of asexual reproduction in the swarming dynamics of the species during times of increased oceanic primary productivity (Kremer, 2002, Henschke et al., 2013)
At thepresent time an abundance of research is available describing the asexual reproduction of T. democratica. However, anatomical information describing the process of formation and development of T. democratica buds is deficient. Sköld et al. (2009) identifies the site of asexual budding as simply anarea near the heart, while other research identifies the site no more specifically than ‘surrounding the stomach’ (Miller and Cosson, 1997). Therefore the aim of this research is to identify if possible, the origin point of bud formation within solitary T. democratica individuals.
METHODS
Collection Methods:
T. democratica swarms were collected in plankton tows carried out on the Eastern side of island over the continental shelf area. Upon collection, salps were transferred into a 4 litre bucket containing fresh seawater until transported to the laboratory where they were maintained in a 50cm x 25cm aerated glass tank filled to 25 centimeters with fresh sea water. The first sample was maintained in culture for 36 hours before perishing. The second was maintained for 24 hours before being released back to the ocean water.
Preparation of organisms:
Solitary individuals were removed from the tank using a large ended 1ml pipet (end cut to increase diameter) and placed individually into a 12-well plate and fixed using a solutionof 4% paraformaldehyde (PFA) in seawater. Samples were placed in the fridge for 4 hours and removed. PFA solution was drained and the animals were rinsed with freshwater 3 times. The wells containing fixed animals were then filled with freshwater and sealed using parafilm until required for slide preparation.
Whole animal slide preparation:
Whole animals were stained using DAPI and Pheloidin stains . Animals were suspended within the staining solution for 30 minutes. Animals were then prepared on a 25mm x 75mm microscope slide. A small amount of ProLong Gold was applied to the animals prior to the application of the cover slip.
Clonal mass slide preparation:
Clonal masses were extracted from whole animals after fixation. They were removed by dissection using micro dissecting needles and fine tipped forceps under a dissection microscope. After extraction of the clonal mass, the samples were prepared on microscope slides in the same manner as the whole animal preparations.
Microscopy:
Prepared slides were analyzed a fluorescence microscope. Normal light (without fluorescence) images were taken, followed by fluorescence images of DAPI stained nucleated cells and Philoidin stained muscle cells.
RESULTS AND DISCUSSION
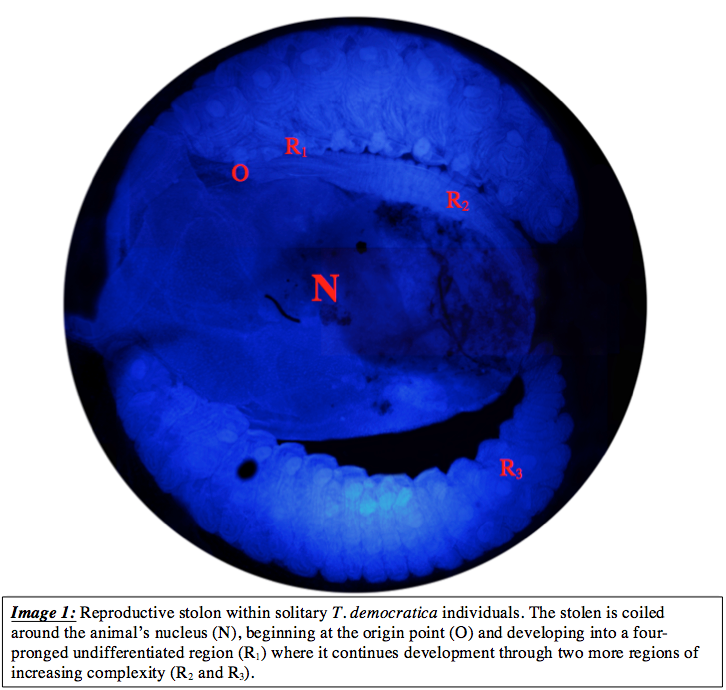 Microscopy of the reproductive stolon identified a number of morphologically distinct sections along the length of the chain. For the sake of simplicity, the stolon was thus regionalized into 3 regions of distinct stages of development, defined as R1, R2, and R3. Located within the posterior ventral region of the solitary T. democratica, the stolon was observed in a coil formation tightly surrounding the nucleus (image 1). Due to this coiling, it was suspected that an origin point must be present within the coil signifying the initiation point of asexual reproduction. Due to the nature of cloning, which takes place within solitary T. democratica individuals, the point of origin was assumed to be the area which would contain the stem cell/s from which the clonal aggregates are formed. Identified as O in image 1, the point of origin is seen where the stolon intersects and joins onto the nucleus, however, more specific microscopy was needed to find the exact point on the nucleus from which the stolon arose. Microscopy of the reproductive stolon identified a number of morphologically distinct sections along the length of the chain. For the sake of simplicity, the stolon was thus regionalized into 3 regions of distinct stages of development, defined as R1, R2, and R3. Located within the posterior ventral region of the solitary T. democratica, the stolon was observed in a coil formation tightly surrounding the nucleus (image 1). Due to this coiling, it was suspected that an origin point must be present within the coil signifying the initiation point of asexual reproduction. Due to the nature of cloning, which takes place within solitary T. democratica individuals, the point of origin was assumed to be the area which would contain the stem cell/s from which the clonal aggregates are formed. Identified as O in image 1, the point of origin is seen where the stolon intersects and joins onto the nucleus, however, more specific microscopy was needed to find the exact point on the nucleus from which the stolon arose.
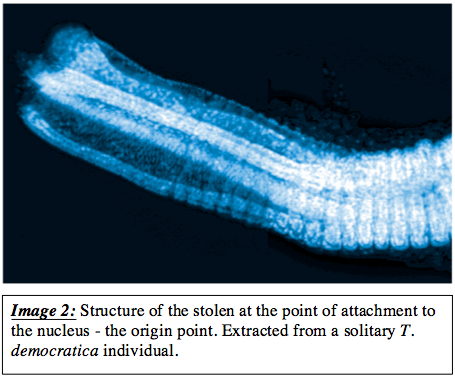
Region 1 is comprised of tissue that appears indistinctly differentiated in comparison to regions 2 and 3. This section, extending from the point oforigin incorporates a rigid, 4 pronged structure that attaches tothe nucleus. This structure forms a square-like aperture,with each ‘prong’ acting as a corner structure connecting to adjacent ‘prongs’ by highly nucleated tissues (image 2).
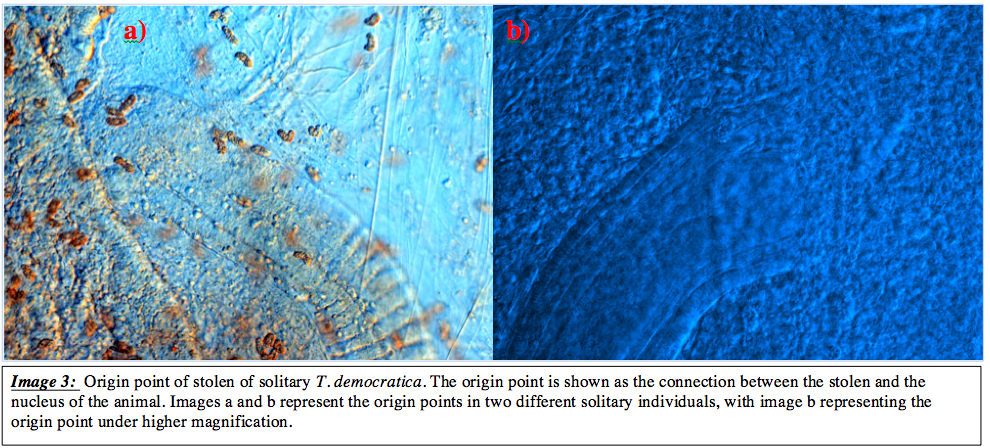
Isolation of the entire reproductive unit allowed the identification of the origin point of the stolon. Both origin points were
located laterally on the nucleus, however the sides and more specific regions from which the stolons originated varied among all individuals analysed. This point is identified in image 3. It remains unknown whether the specific area identified here contains the stem cells that give rise to the zooids. Unfortunately more intensive analysis would be necessary to identify such cells, which was not performed in this study. However, it does provide abasis for further investigation into the asexual reproduction in T. democratica.
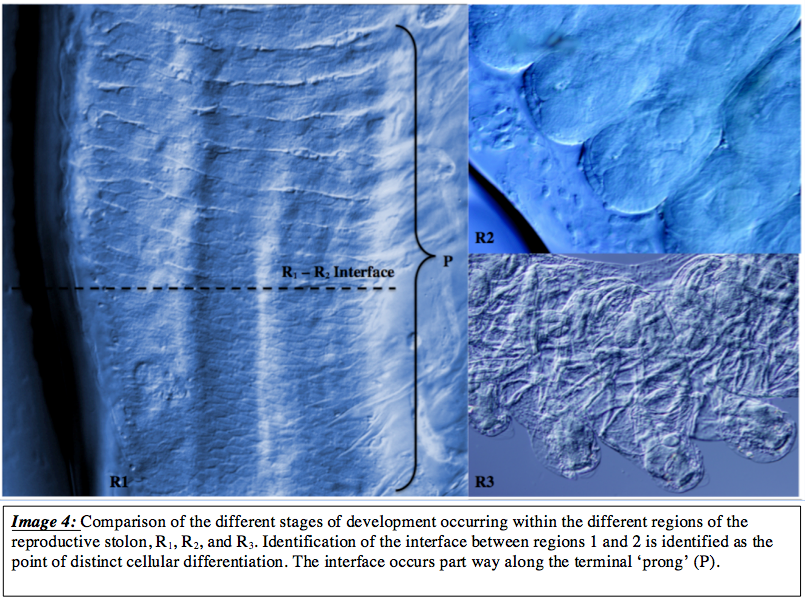 Extraction of a zooid chain allowed more specific morphological analysis of the developing sexual individuals. Physical changes, whic
h occur throughout the developmental process, are complex and occur over a very short time scale. Between the initial differentiation from the unsegmented R1 region cells, development intocomplete zooids of 1.1 – 2.1mm in length, may occur in less than 24 hours depending on environmental conditions (img 3) ( Deibel and Lowen, 2012, Deibel, 1982). The sharp interface between R1 and R2 regions was particularly interesting, shown here in image 3, as it indicates a region of developmental stimulation. The stimuli is unknown and has not been documented in current literature, however the sudden progression from somewhat smooth tissue with ill-defined differentiation to the well differentiated transversely segmented tissues would infer that a developmental cue is initiated in this region, whether by endogenous signalling molecules such as that observed in Planarian stem cells ( Shibata et al., 2010) or by a combination of biochemical and physical cues such as that in some human stem cells ( Chaudhuri and Mooney, 2012). 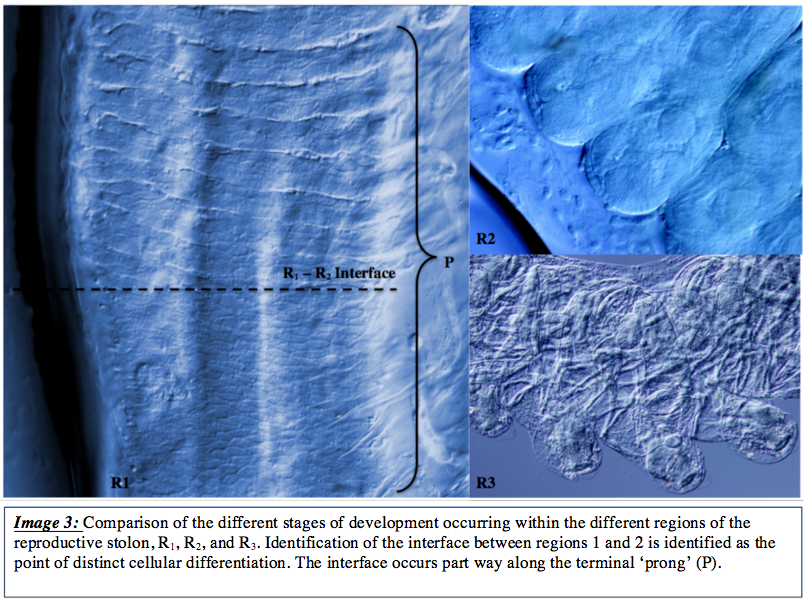
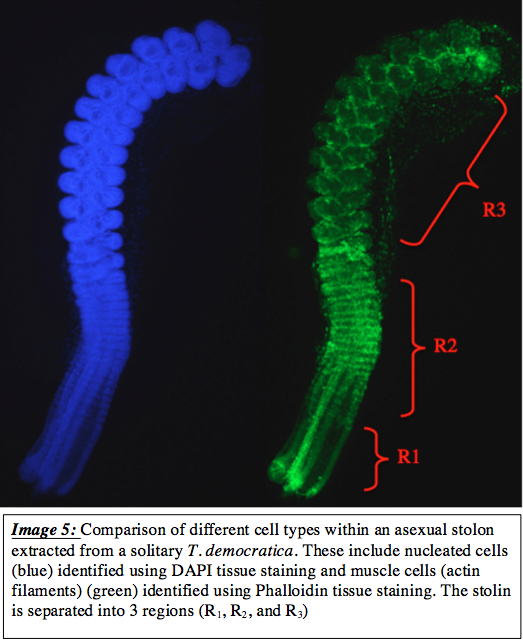
Throughout earlier development, the cellular composition of the stolon does not change significantly with respect to cell types, and consisted most heavily of nucleated cells (image 5). A far smaller number of muscle cells were present during these early stages, however, these were mostly concentrated around the attachment strands between the zooids ranther than within the zooids. This indicates that the formation of muscle occurs much later in development than shown in image 5.
Analysis of the cellular composition of more developed zooids indicates that muscle cells are prominent, and organised into distinct bands in these later stages (image 6).From this it may be infered that the development of muscles occurs towards the latter stages of development, later than other structures such the anterior eye spot and dorsal tubercle which were documented present in earlierstages than muscle bands.

|